
HOME > Research
Nghiên cứu lâm sàng
Human Clinical Study Ⅰ(9):
Thí nghiệm ở người Ⅰ(9):
Kobayashi et al. (1991) asked 16 terminal cancer patients (Table 2) to consume S100 Milk tablets (3-18 tablets, about 0.3g per tablet) for two months, and then they evaluated the satisfaction index of the patients during the study and the changes in the clinical index shown by the QQL (Quality of Life) scale. After taking the S100 tablets for one month, 30.8% of the patients felt that the milk tablets were helpful and 15.4% of the patients thought their bodies were significantly improved. After taking for two months, up to 50.0% of the patients thought their bodies were significantly improved. The overall improvement rate was as high as 60% (Fig. 7)(9).
Improvements in clinical symptoms due to cancer included: 37.6% of the patients thought the symptoms of abdominal distension and pain were relieved; 31.3% thought that systemic lassitude was improved; 26.7% thought that constipation was reduced; 25.1% thought the feeling of nausea and vomiting was reduced; 25% thought conditions including high fever, sweating and loss of appetite were improved(9).
Kobayashi (1991) tiến hành thí nghiệm với 16 bệnh nhân ung thư (Bảng 2) sử dụng sữa S100 loại viên nén (3-18 viên, 0.3g/viên) trong 2 tháng, Sử dụng QQL (Quality of Life) để đánh giá mức độ hài lòng của ngưởi bệnh trong thời gian điều trị. Sau 1 tháng sử dụng S100, 30.8% người bệnh cảm thấy có hiệu quả với sức khỏe, 15.4% bệnh nhân nhận thấy sức khỏe có cải thiện đáng kể. Sau hai tháng sử dụng 50% người bệnh nhận thấy sức khỏe được cải thiện (hình 7) (9).
| What kind of cancer do subjects suffer from? | ||||
| Name of diagnosis (Tên bệnh) | Subjects (Số lượng) | |||
| Breast cancer (Ung thư vú) | 8 | |||
| Gastric cancer (Ung thư dạ dày) | 1 | |||
| Uterine cancer (Ung thư tử cung) | 1 | |||
| Esophageal cancer (Ung thư thực quản) | 1 | |||
| Renal cell carcinoma (Ung thư tế bào thận) | 1 | |||
| Rectal cancer (Ung thư trực tràng) | 1 | |||
| Lung cancer (Ung thư phổi) | 1 | |||
| Pancreatic cancer (Bệnh ung thư tuyến tụy) | 1 | |||
| Chondrosarcoma (Bệnh chondrocarcoma) | 1 | |||
| Total (Tổng) | 16 | |||
|
Table 2. Cancer types of 16 terminal cancer patients(9). Bảng 2. Các loại ung thư của 16 bệnh nhân ở giai đoạn cuối (9). |
||||
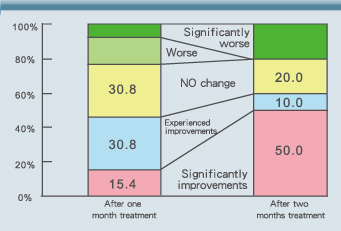
Fig. 7 After taking S100 Milk tablets for one month, 30.8% of the subjects thought they experienced improvements; 15.4% thought the improvements were significant. After taking for two months, 10% of the subjects felt there were improvements; 50% thought there were significant improvements(9).
Hình 7. Sau khi sử dụng S100 viên nén một tháng, 30.8% tình nguyện viên có cải thiện sức khỏe; 15.4% sức khỏe cải thiện đáng kể. Sau hai tháng sử dụng, 10% số tình nguyện viên có cải thiện sức khỏe, 50% tình nguyện viện có sức khỏe cải thiện đáng kể (9).
There were also improvements shown in blood chemistry indexes. The levels of RBCs, Hb and Hct in subjects all increased significantly after they took the S100 Milk tablets reinforcing what was reported by the subjects themselves(9).
Tổng kết kết quả thí nghiệm như sau: có 37.6% triệu chứng đau ngực, tức ngực có dấu hiệu cải thiện. 31.3% bệnh nhận sức khỏe cho rằng sức khỏe của họ có thể cải thiện ,26.7% bệnh táo bón giảm; 25.1% có triệu chứng nôn mữa, 25% sốt cao, đổ mồ hôi, ăn không ngon được cải thiện.
Những chỉ số sinh hoa trong máu cũng được cải thiện, mức độ RBCs, Hb, Hct tăng sau khi tình nguyện viên sử dụng sữa S100 (9).
Human Clinical Study Ⅱ(10) :
We understand that regular exercise is beneficial for overall health. However, prolonged and intense physical activity may have adverse effects on the body, potentially leading to a decrease in immunity, induction of inflammatory responses, and subsequent harm to muscles and organs. It may also result in gastrointestinal symptoms such as bloating, abdominal pain, diarrhea, vomiting, and erosive gastritis.
In a clinical human trial conducted in Japan (Ma et al., 2020), researchers recruited seven healthy young individuals who were randomly divided into two groups. One group consumed immune protein powder (IMP), while the other group consumed regular protein powder (placebo protein, PLA). Participants ingested the assigned product twice daily, with each serving containing 10 grams, for 8 weeks. Following this period, a 1-week washout period was implemented, and then the groups switched products for an additional 8 weeks. Before product use (M0), after 1 month of product use (M1), and after 2 months of product use (M2), participants underwent a 3000-meter sprint test, and relevant indicators were measured.
Intestinal Fatty Acid Binding Protein (I-FABP) is an indicator used in hospitals to assess intestinal damage. The experimental results demonstrated a significant increase in I-FABP after intense exercise, indicating damage to the intestines. However, the group supplemented with IMP showed a significant reduction in the increase of I-FABP caused by intense exercise (refer to Fig. 8). This confirms that immune protein powder effectively protects intestinal health during strenuous physical activity(10).
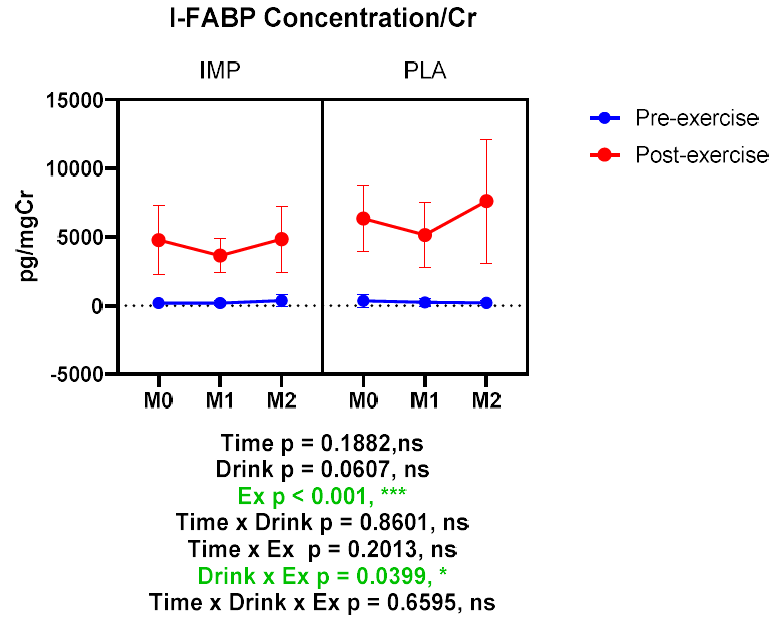
Fig. 8. I-FABP concentration corrected for creatinine. Data are expressed as mean ± standard deviation.
* p < 0.05 和*** p < 0.001. Green words indicate significance<0.05 (10).

